Citation:
Abstract:
In November 2020 as the world grappled with a over 50 million cases of Coronavirus disease 2019 (COVID-19) and over 1 million deaths, all eyes were focused on the development and manufacturing of COVID-19 vaccines. This case traces the complex set of activities involved in the development, regulatory approval, production, and distribution of COVID-19 vaccines. It describes the resources, information, cooperation, and decision making that went into this unprecedented effort. It poses some of the difficult strategic and operational decision dilemmas faced by country governments, purchasers, vaccine developers, and manufacturers in designing the overall supply chain for COVID-19 vaccines.
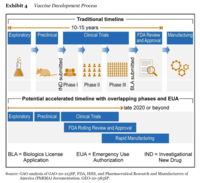
Learning Objectives: A productive class discussion will allow students to appreciate the development, regulatory approval, production, and distribution processes for a pandemic vaccine; The complexity and inter-relationships between different activities and actors in the vaccine supply chain; The role of manufacturing flexibility in managing uncertain demand and supply; and the importance of a portfolio approach in sourcing/purchasing technology which is under development.
Keywords: product development, supply chain management, manufacturing, public-private partnerships, health care delivery, public health, vaccine development, clinical trials, pandemic response, COVID-19